在之前的考试测验中,同学们都对的自己的分数有疑问,明明答对了,意思到位了,就不给分,有的还倒扣分了,觉得非常不服气和委屈。
首先,我想说老师批的严格,才能引起学生们重视。试卷上不是仅仅写的意思对就行了,还要答到得分点。然后,等同学们真正参加CIE 考试时就会避免这个问题。所以,请同学们一定要正视这个得分点。目前,希望同学们需要调整好心态,继续努力复习,常考知识点多扫几遍,加油!
下面给同学讲解一份2017年的CIE真题,结合Mark scheme讨论一下不得分的原因和注意点,希望能为CIE的考生们尽点绵薄之力!
1.The composition of atoms and ions can be determined from knowledge of atomic number, nucleon number and charge.
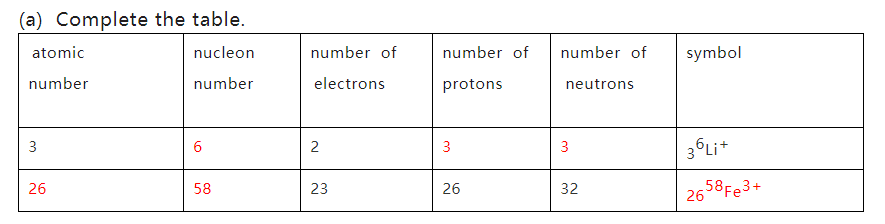


(i) Define the term relative isotopic mass.
The mass of a particular isotope of an element on a scale in which a C-12 atom has a mass of exactly 12 units
解析:定义需要记忆,一定是C-12的质量作为标准(不能是C)mass of an atom得一分;on a scale in which a C-12 atom has a mass of exactly 12 units 得一分
(ii) Calculate the relative isotopic mass of 11B.Give your answer six significant figures.Show your working.
10.0129×19.78%+80.22%X=10.8(periodic table里面找)
X=10.9941 {total:6}
解析:atomic mass乘以各自对应的abundance等于average atomic mass就行简单计算题,类似的计算,同样的做法。
2.Nitrogen gas, N2, is very unreactive.
(a) Explain why nitrogen gas is so unreactive.
The triple covalent bond in N2 is very strong;its bond energy is almost 1000 kJ/mol. It is difficult to break and so nitrogen gas will not react under universal condition ,just like noble gases are non-polar molecules.
解析:strong triple bond 得一分;non-polar/no dipole 得一分(不太合理的得分点,活泼性和极性关系不大)
(b) Despite the low reactivity of N2,oxides of nitrogen occur in the atmosphere through both natural and man-made processes.
(i) Explain why oxides of nitrogen can be produced by internal combustion engines.
nitrogen and oxygen from the airreact each other under: high temperature (of internal combustion engine)/(engine) produces enough
解析:在极端条件下N2和O2才会发生反应
(ii) State and explain, using a suitable equation, how oxides of nitrogen produced by internal combustion engines can be prevented from reaching the atmosphere.
In catalytic converter ,reduction reaction take place 2NO + 2CO → 2CO2 + N2(decomposition of NOx using a catalyst) [2]
解析:写出 equation 得一分,还原反应在 catalytic converter 里面发生 得一分
(iii) State the role of nitrogen dioxide, NO2,in the formation of acid rain by oxides of sulfur.Write suitable equations to explain this role.[3]
role acts as a catalyst OR oxidising agent 得一分;equation 1 SO2 + NO2 → SO3 + NO 得一分;equation 2 NO +½O2 → NO2 OR SO3 + H2O → H2SO4 得一分
解析:acid rain的形成和H2SO4 、HNO3有关系,和CO2无关,因为H2CO3是弱酸
(iv) Suggest an equation to show how NO2 can contribute directly to acid rain.
2NO2 + H2O → HNO2 + HNO3 OR 4NO2 + 2H2O + O2 → 4HNO3
解析:注意写好equation还要配平,不配平扣分会很可惜
(c) Explain how the uncontrolled use of nitrate fertilisers on land can lead to a severe reduction in water quality in rivers.
1. a bloom of algae can spread across the surface ,blocking out the light for other plant life in the water
2. When the plants and algae die ,bacteria in water feed on them ,decomposing the plant material.
3. The bacteria multiply rapidly with so much food available, using up the dissolved oxygen in the water .
4.So drop inconcentration of oxygen, fish can not extract enough dissolved oxygen from water,Without this dissolved oxygen,they die, affecting the whole ecosystem.
{3} {total:13}
解析:这里的reduction是下降不是redox reaction中还原的意思。红色部分是得分点,得分点中的专业术语都是频繁出现的,比如:decompose/break down,很多同学大考的时候都用自己的话表达出正确的意思而没得到应得的分数,就是用词还不够专业。
3.The hydrogen halides, HCl, HBr and HI, can undergo thermal decomposition.
In a sealed container an equilibrium is established according to the equation shown.
2HX(g) ⇌ H2(g) + X2(g) (where X = Cl, Br or I)
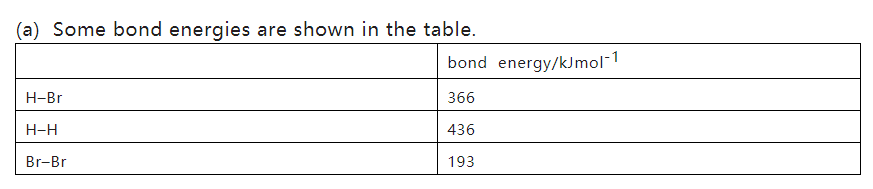
Use these data to calculate a value for the enthalpy change, ΔH, for the thermal decomposition of hydrogen bromide, HBr, according to the equation shown.
Reactant bond energy-product bond energy= 2×366-(436+193)= 103 KJ/mol [1]
ΔH = ............................kJ/mol
解析:1.Bond个数不要数错,Reactant中bond break是endothermic的过程,form bond of product是exothermic的过程,反应物吸热比产物放热的bond energy大,所以整个reaction的ΔH>0,是吸热反应。
2.ΔH>0对应endothermic ,ΔH<0 对应exothermic
(b) At a temperature of 700K a sample of HBr is approximately 10% decomposed. Changing the temperature affects both the rate of decomposition of HBr and the percentage that decomposes.
The Boltzmann distribution for a sample of HBr at 700K is shown. Ea represents the activation energy for the reaction.
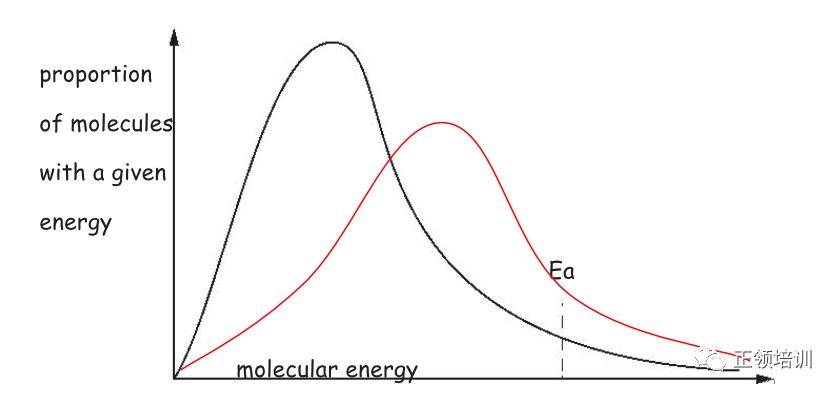
(i) Using the same axes, sketch a second curve to indicate the Boltzmann distribution at a higher temperature.
解析: peak are displaced to right of original and starts at origin 得一分
the peak is lower and curve crosses once only finishing above original 得一分
(ii) With reference to the curves, state and explain the effect of increasing temperature on the rate of decomposition of HBr.
The increasing temperature increased energy /rate results in particles moving around more quickly,which increases the frequency of collisions per unit time . the proportion of successful collisions increases because the proportion of particles exceeding the activation energy((E≥Ea))increases.This is the more important factor.
解析:increasing temperature increased energy/rate 得一分;E≥Ea 得一分;higher frequency of successful collisions per unit time 得一分。
温度越高,反应速率常数k=Ae-Ea/RT 越大 [3]
(iii) The decomposition of HBr is endothermic.
State the effect of increasing temperature on the percentage of HBr that decomposes.Use Le Chatelier’s principle to explain your answer.
When the temperature decreasing, the equilibrium shifts to the left/in the forward direction/endothermic direction .The decomposition of HBr will decreases.[3]
解析:正反两个方向要说全,才能得三分。如果改变reversible reaction的条件(如浓度、压强、温度等),equilibrium就被破坏,并向减弱这种改变的方向移动。
(iv) At 700K HBr is approximately 10% decomposed but hydrogen iodide, HI, is approximately 20% decomposed.Explain this difference with reference to bond strengths and the factors that affect them.
Because H-Br(0.141nm)bond strength is less than H-I(0.161nm). Bond energy of H-I is smaller than bond energy of H-Br. Iodine atom is bigger than bromine atom, so attraction force of nucleus in iodine for shared pair of electrons is weaker then bromine. [3]
解析:HBr比HI键长短键能大,所以相同温度下,较难分解。注意红色标记的得分点。
(c) At temperatures above 1500K, HCl will decompose.A sample of 0.300mol of HCl decomposed in a sealed container.The resulting equilibrium mixture wasfound to contain 1.50 × 10-2mol of Cl2
(i) Calculatethe amounts, in mol, of H2 and HCl present in the equilibrium mixture.

(ii) Calculate the mole fraction of each gas inthe equilibrium mixture.
0.27mol/0.3=0.9
0.015mol/0.3=0.05
0.015mol/0.3=0.05
mole fraction of HCl = ....0.9...............
mole fraction of H2 =...0.05...............
mole fraction of Cl 2 = ....0.05.............[1]
解析:要计算反应粒子数,根据反应方程中的系数,对应成比例即可。简单计算
(d) In another experiment under different conditions, an equilibrium mixture was produced with mole fractions for each species as shown.
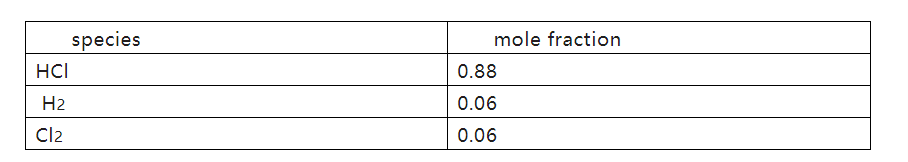
(i) Write the expression for the equilibrium constant, Kp,for the decomposition of HCl.
2HCl(g) ⇌ H2(g) + Cl 2(g)
Kp = PH2·PCl2 / PHCl2
解析:注意是PHCl的二次方,不是两倍PHCl
(ii) Explain why the total pressure of the system does not need to be known for Kpto be calculated for this experiment.
The total pressure is related to the mole of particles,not the Kp.
解析:Kp只和温度有关系,是温度的函数[1]
iii) Calculate the value of Kp for this experiment.
Kp = PH2·PCl2 /PHCl2
=0.06×0.06/(0.88)2
=4.649×10-3
解析:数字不要代入错,也是简单计算题。
Kp = .............................. [1] [Total: 18]
4. (a) The hydrocarbons A, C4H10,and B, C4H8, are both unbranched.
A does not decolourise bromine.
B decolourises bromine and shows geometrical isomerism.
(i) Draw the skeletal formula of A.
(ii) The hydrocarbon A, C4H10, has a branched isomer.Suggest why unbranched A has a higher boiling point than its branched isomer.
The straight chain can line up beside each other so there are a large number of contact points. It has more temporary dipole-dipole force and larger van der waals’ force. The unbranched molecule need more energy to break the intermolecular forces. The van der waals’ force are higer , so the boiling point is higer .
解析:因为分子间接触面积大,temporary dipole-dipole force就会大。 [2]
(iii)Give the structural formula of B.
CH3CH=CHCH3 [1]
解析:structural formula可以省略C-Hbond,最好写出functional group “=”他能使得bromine water(根据浓度不同yellow、orange、red-brown)褪色(常考考点)
(iv) Explain why B shows geometrical isomerism.
No rotation/restricted/limited rotation of C=C/(carbon) double bond 得一分;One (of the two) methyl groups/one (of the two) H (atoms) is on each C (of C=C) [2]
(v) Draw the mechanism of the reaction of B with bromine, Br2. Include all necessary charges, dipoles, lone pairs and curly arrows. [4]
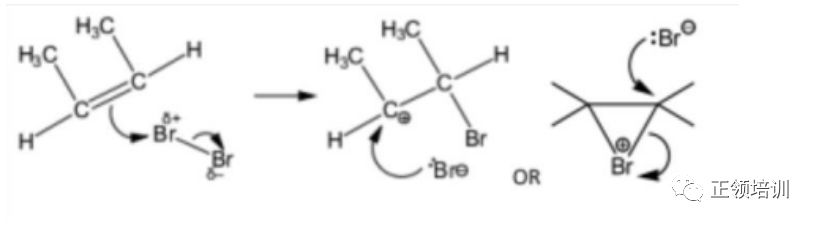
解析:The driving force for this reaction is the formation of an electrophile X+ that forms a covalent bond with an electron-rich unsaturated C=C bond. The positive chargeon X is transferred to the carbon-carbon bond, forming a carbocation during the formation of the C-X bond.
(vi) Explain the origin of the dipole on Br2in this mechanism.
The high electron density in double bond repels electrons away from nearest Br. [1]
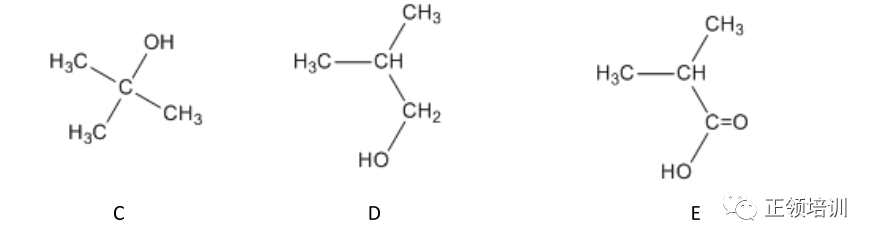
(b) The alcohols C and D are isomers of each other with molecular formula C4H10O.Both isomers are branched.
When C is heated under reflux with acidified potassium dichromate(VI) no colour change is observed.
When D is heated under reflux with acidified potassium dichromate(VI) the colour of the mixture changes from orange to green and E, C4H8O2,is produced.
E reacts with aqueous sodium carbonate to form carbon dioxide gas.
(i) Identify C, D and E.
(ii) Write the equation for the reaction between E and aqueous sodium carbonate.
2C4H8O2 + Na2CO3 → 2C4H7O2Na + H2O + CO2 [1]
解析:能和Na2CO3 反应的-COOH、-SO3H、C6H5OH
(c) The isomers F and G, C5H10O,both form an orange precipitate when reacted with 2,4-DNPH.F is unbranched and reacts with alkaline aqueous iodine to produce a yellow precipitate.G does notreact with alkaline aqueous iodine. It contains a chiral centre and produces asilver mirror when warmed with Tollens’ reagent.
(i) Name the yellow precipitate produced by the reaction between F and alkaline aqueous iodine.
Triiodomethane 解析:tri表示三,iodine去掉ine加o [1]
(ii) Give the structural formula of F and of G.
F CH3CH2CH2COCH3
G C2H5CH(CH3)CHO
解析:aldehyde and ketone都能和2,4-DNPH反应,因为Gdoes not react with alkaline aqueous iodine,F is unbranched and reactswith alkaline aqueous iodine。所以F中含有-OCH3 group [2]
(iii) Explain the meaning of the term chiral centre.
The carbon atom with four different groups attached is called the chiral centre of the molecule. [1]
解析:A chiralcenter is a stereocenter consisting of an atom holding a set of ligands (atomsor groups of atoms) in a spatial arrangement which is not superimposable on its mirror image. 得分点:one atom with four different groups
(d) H and I are isomers with molecular formula C2H4O2.The infra-red spectra of isomers H and I are shown.
(i)Identify the bonds responsible for the principal peaks above 1500 in each spectrum.
spectrum of H C=O bond ANDO–H bond
spectrum of I C=O bond ANDC–H bond
解析:O-H伸缩振动吸收位于~3550cm-1处,C=O伸缩振动位于~1760cm-1处
Ester中的C=O在1715-1750cm-1,C–H 在2850cm-1 [2]
ii) Name H and I.
H ethanoic acid
I methyl methanoate
解析:根据formula和group写出name [2]
[Total: 23]
欢迎读者和同学们批评指正、留言交流!CIE化学考试就在眼前,同学们加油!




